Ribbel International Limited
Offering good quality Disposable Surgical Products, immensely used by the surgeons in the health care industry...

Most Popular Products
Striking Features:
- Anti-corrosive surface finish
- Suitable for surgery
- Fine finish
- Blunt edges
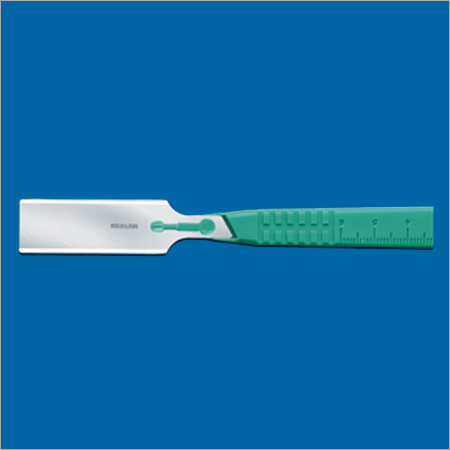
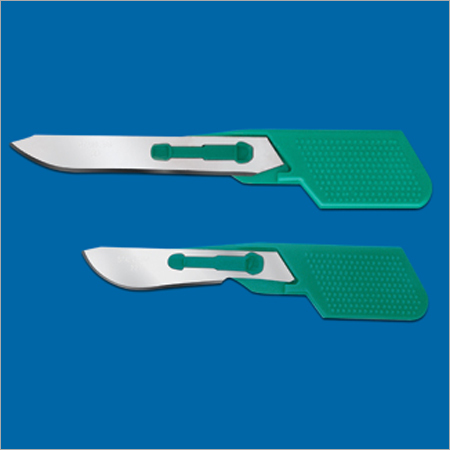
An ISO 9001:2000 certified company, Ribbel International Ltd. is the manufacturer, exporter and supplier of a wide range of disposable surgical products. Using only a superior quality raw material, our immaculate product range includes Surgical Blades (in high carbon and fine stainless steel), Disposable Surgical Blades, Surgical Instruments, Disposable Disposable Scalpels, Thumb Scalpels, Stitch Cutters, Post-mortem Knives, Skin Graft Blades, Blood Lancets and Blade Removers have carved out an impressive presence in the markets today.
Company Profile
A state-of-the-art manufacturing process, under a stringent quality control and sterile environment defines our admirable product line. We implement collaborative efforts for assuring quality at its best, in our products and services. Owing to avant-garde nature of our solutions, we have acquired respected client base in various countries. These are Argentina, Brazil, Canada, Germany, Holland, Italy, Mexico, Portugal, U.K., U.S.A., Middle East, and South East Asia. As our success mantra is concerned, we persistently strive to add new customers to our list.
We, Ribbel International Ltd.
is an ISO 9001 certified organization which is engaged in
manufacturing a broad range of disposable surgical products that are
immensely used in hospitals, research centers, medical institutes,
dispensaries, medical health centers etc. All these surgical items are
made up of high grade components by using the latest technology in tune
with international quality standards and norms. As our entire range of
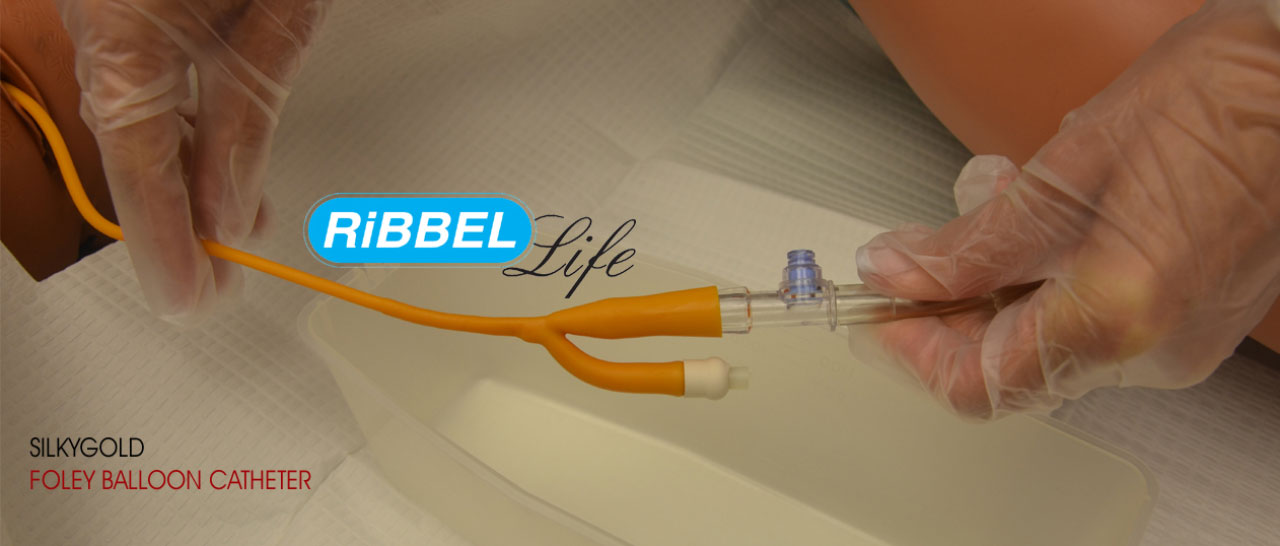
Foley Catheter, Disposable Scales, Thumb Scalpels, Stitch Cutters etc.
are used for surgery purposes, we make sure that they are produced in a
clean and hygienic environment. With the support of following
knowledgeable key members, we are able to earn a prestigious reputation
in the market:
- Mr. Avinash Kanodia, Director (MBA- USA Marketing)
- Mr. R.K. Kanodia Promoter / Managing Director
- Mr. Vikram Kanodia, Director (MBA- USA, International Sales)
- Mrs. Suman Kanodia, Promoter/ Director
- 1992 : With the concept to produce & export premium quality surgical blades in Europe, Kanodia Family laid their foundation.
- 1994 : The company is started producing surgical items.
- 1995 : The demand and sale of goods are increased in Europe & India.
- 1996 : We started OEM business in North America.
- 1998 : Achieved ISO 9001 certification from DNV, Norway
- 1999 : Attained the certification of EN46002 and EC by DNV, Norway
- 2004 : Became ISO 13485 certified company & launched different kinds of blade sizes as per customer requirements.
- 2007 : Entered into the business of manufacturing Syringe at Haridwar plant for Indian Market & later in 2013, converted the business into producing Respiratory care medical devices such as Nebulizer etc.
- 2009 : Developed wide stretched distribution network which is covering around 65 countries.
- 2012 : Enhanced the production capacity of Blades by acquiring another local blade manufacturer in India.
- 2013 : Introduced Respiratory Care medical devices.
- 2014 : Established new manufacturing plant of Foley Catheter which is equipped with world class facilities.
- 2015 : Have Double Blade manufacturing capability and today, attained the position as the largest blade manufacturing company in India.
Product Quality
We
have developed in-house testing laboratory where our whole assortment
of surgical items are underwent through rigid quality testing
procedures. Our
every single product is thoroughly examined by the quality experts to
make sure that they are free from any kind of flaws or defects. All the
standard fitting surgical blades and scalpels are fabricated in
accordance to BS 2982 1992 / ISO 7740 1985 / EN 27740 1992 standards.
Our organization is accredited by EN 46001 and allowed by Medical Device
Directive 93 / 42 / EEC to utilize CE mark on our products.
Another
reason that our surgical goods are demanded extensively in the market
is due to our ISO 9001 certification which is approved by DNV (Det
Norske Veritas) of Rotterdam, Holland and CE marking which is certified
by DNV (Det Norske Veritas) Norway. Furthermore, all our surgical items
are also registered under FDA of USA, TGA of Australia and MOH in Russia
& Brazil.
We
are the leading manufacturer, exporter and supplier of a wide gamut of
disposable surgical products. These are broadly defined as:
- Disposable Syringes (3 Parts)
- Surgical Blades (in High Carbon and Fine Stainless Steel)
- Foley Catheter
- Disposable Scales
- Thumb Scalpels
- Stitch Cutters
- Skin Graft Blades
- Blood Lancets
- Gauge Blades
- Fine Blades
- Microtomes
- Biopsy Punches
- Blade Removers
- IV Sets.
Our Infrastructure
Our
company is backed with cutting-edge technology and hi-tech equipment that are
specially imported from Germany which helps us to produce superior quality
surgical items. By optimally utilizing these sound
facilities, we are able to upgrade our rate of production to 50 Million
blades per annum and enhance the overall quality of the end products.
To
set a certain benchmark for our products, we produce them in compliance
with BS2982 and Bard Parker FDA USA interim standards. Apart, our
production personnel use the most effective hardening processes to
sharpen and to provide extra strength to blades.
Technological
advance Automatic Grinding Machine is installed in our premise which is
PLC controlled and operated by the technical experts. The polishing and
de burring processes we have adopted provides rust proof surface
finishing and clean cutting edge to the surgical items.
To ensure that our manufacturing facilities are developed in compliance to GMP, several periodic audits are carried out by the professional body of DNV. Being an ISO 9001 certified manufacturer, we carefully fabricate our products that are carrying the accreditation of CE 0434 mark. Our quality management team thoroughly inspect all the production facilities by conducting bio-burden and sterility tests in the appropriate manner. All the operations related to inspection and packaging are takes place in 100,000 certified clean rooms.
Traceability & Accessibility of Records
We have provided LOT numbers to the units packs and foil packs to easily identify them during the process of dispatch and distribution. Proper records are prepared and maintained by the personnel so that they can trace easily throughout the supply & distribution chain.
Accreditations
A Distributor from Germany wrote- Please note that we are extremely happy about the last shipments from your side as the products are of top quality and delivered on time. And after these shipments you are our supplier no. 1 for blades.
An End user wrote (Dr.T.Krishnamurthy, Kavery medical centre and hospital, Tiruchirapalli 620018) - We received the blades and used the carbon blades yesterday. The blades were of very good quality and it was a pleasure to use the same.
"I am satisfied with quality especially sharpness of the blade."
"I would rate this product as one of the best manufactured in out country."
Dr. Mohan A. Joshi Ms. FCPS. FAIS
Associate professor of Surgery.
Consultant Surgeon. Therapeutic Endoscopes.
Jt. Secretary Gastroenterology Research Society, Mumbai.
Governing Council Member of Society of Gastrointestinal Endoscopes of India.
L.T.M Medical College Sion. Mumbai 400022
"I have used them and appreciated the quality of blade very much."
Shekhar Nursing Home
Panchenda Road,
Muzaffarnagar.
"I have since used them and found to be quite good"
Dr. M. Baskaran Selvapathy
M.S. M.N.A.M.S., F.I.C.S.
Civil Surgeon Madras
"Blade are simply superb and highly dependable."
Prof. Dr. V. Panchamoorthy.
M.s. (Gen. Surgery), M.ch. (Thoracic Surgery), FCCP., FICS.,FICA.,
FMMC., FACC (Cardiology)., F.I.A.M.S., F.I.A.C.S.,
former Professor of Cardio-Thoracic Surgery, Madras Medical College,
Cardio Thoracic Surgeon, Govt. General Hospital, Madras.
Chief Cardio-Thoracic Surgeon.
We are blessed with sound in house testing laboratory where our complete gamut of surgical items are passed through stringent quality tests...
Our company is supported with world class technology and equipment that are specially imported from Germany so as to provide premium quality...
A Distributor from Germany wrote- Please note that we are very happy about the shipments from you the last time regarding delivery and quality...
 |
RIBBEL INTERNATIONAL LIMITED
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |